Which of the Following Has No Net Dipole Moment
1-Which of the following has no net dipole moment. In non-polar molecule the centres of positive and negative.

Solved 1 Which Of The Following Has No Net Dipole Moment O Chegg Com
Which of the following alkanes has no net dipole moment.
. Upon which of the quantities is the magnetic dipole moment of a. O CHCI O NF3 N20 TeO HSe check_circle Expert Answer. Which of the following does not have a molecular dipole moment.
C H C l 3 has net dipole moment as the bond dipoles do not cancel each other. 1 current 2 resistance 3 coil area 4 wire cross-sectional area and 5 magnetic field. Electronegativity in halogens decreases from top to bottom.
Which of the following has a net dipole moment. As a result the CO 2 molecule has no net dipole moment even though it has a substantial separation of charge. But here in ccl4 its dipole moment is zero because the dipole moment due to all four.
Which of the following has no net dipole moment. CCl4 has zero dipole moment whereas CHCl3 has non zero dipole moment. A molecule which has a symmetrical geometry will.
PH3 HBr CH3OH CH3CH3 CH3CH3 The normal boiling point for H2Te is higher than the normal boiling point for H2Se. Which of the following has no net dipole moment. Why is CCl4 not dipole.
95 130 ratings play-rounded-fill. Which of the below molecules has no net dipole moment. Among all the given options TeO 3 has no net dipole moment because the central atom has three bonded atoms one bond being a double bond and has no lone pairs and the geometry is.
Want to see the step-by-step answer. Which of the following has no net dipole moment. Answered Aug 28 2021 by MehulKumar 187k points selected Aug 30 2021 by Ritwik.
A molecule which has no net dipole moment is called non-polar. A molecule which has no net dipole moment is called. Correct Answer - BCD cis form have some net dipole moment.
Consider the following quantities. Were trying to figure out which molecules have are sold in diaper woman and which molecules do not have a result in Naipaul moment. CH3F has the highest dipole and is most polar.
And whya CH4 b CHBr3 c F2 dCBr4 e. N2- they the two are an analogous atoms ie same. 2 CCIA 3 BeF24 SO Open in App.
Which of the following molecules is polar. 101Which of the following has no net dipole momentN2O NF3 H2Se TeO3 CH3Cl. In polar molecules that have permanent dipoles the dipoles are strongly attracted due to weak forces that are called as dipole-dipole attractions.
Which of the following molecules does not have a dipole moment. So we have molecules given to us. In contrast the H 2 O molecule is not linear part b in Figure 228.
Andwhya H2O bNF3 cH2Se dTeO3 e CH3Cl2-. This browser does not support the video element. Which of the following has no net dipole moment.
Check out a sample QA. B C D CHCl_3 has net dipole moment as the bond dipoles do not cancel each otherWhereas other molecules such as CH_4 CO_2 and CCl_4 have zero dipole moment as. Whereas other molecules such as C H 4 C O 2 and C C l 4 have zero dipole moment as the bond dipoles completely cancel each other.
A BF3 B NCl3 C H2Se D CH3Cl. NCERT DC Pandey Sunil Batra HC Verma Pradeep. A SCl2 B H2O C CF4 D BrCl.

Which Of The Following Alkanes Has No Net Dipole Moment Youtube

Solved Which Of The Following Has No Net Dipole Moment A Chegg Com

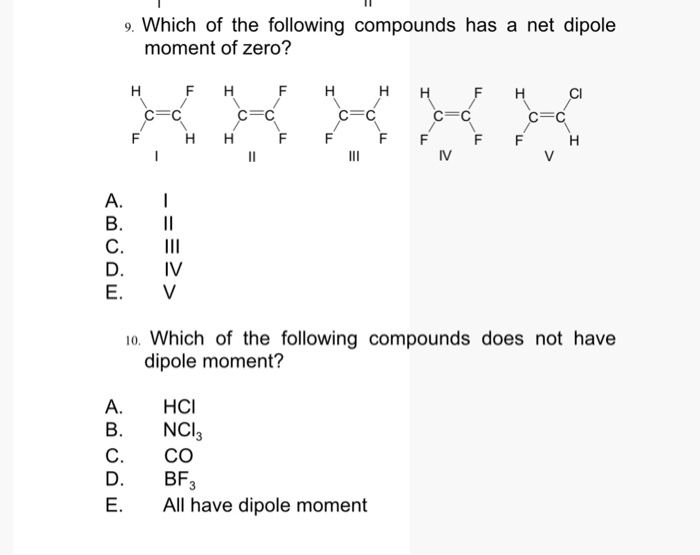
Solved 9 Which Of The Following Compounds Has A Net Dipole Chegg Com


Comments
Post a Comment